
Reacción de BriggsRauscher (Un reloj químico) YouTube
Briggs-Rauscher reaction. The Briggs-Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking color changes: the freshly prepared colorless solution slowly turns an amber color, suddenly changing to a very dark blue.

BriggsRauscher Reaktion Flaskman
The Briggs-Rauscher reaction involving iodate, hydrogen peroxide, malonic acid and Mn(II) in acid solution exhibits bistability and oscillation in a CSTR as well as batch oscillation. We have developed a mechanism for this reaction which reproduces these and other dynamic features. Astonishingly, our model is qualitatively identical to, though.

Black & White Magic (BriggsRauscher Reaction) YouTube
Reaksi Briggs-Rauscher adalah salah satu contoh reaksi osilasi. Reaksi ini cocok digunakan untuk keperluan peragaan karena perubahan warnanya yang mencolok: solusi tak berwarna yang telah disiapkan beberapa saat sebelumnya secara perlahan berubah menjadi warna batu ambar, dan kemudian secara tiba-tiba menjadi biru tua..

BriggsRauscher oscillating reaction Stock Image C021/6618 Science Photo Library
Skripsi ini bertujuan untuk mempelajari reaksi Briggs-Rauscher (BR) yang merupakan reaksi berosilasi. Model reaksi BR oleh De Kepper dan Epstein yang digunakan telah disederhanakan dari 15 variabel konsentrasi dan 10 tahap reaksi sehingga hanya terdiri dari 10 variabel konsentrasi. Konstruksi model menggunakan hukum aksi massa yang memberikan sebuah sistem dari 10 persamaaan diferensial.

X870 BriggsRauscher Reaction Oscillating Clock Lecture Demonstration Manual General
Oscillating magic: the Briggs-Rauscher oscillating reaction. By Declan Fleming 2019-02-25T11:39:00+00:00. No comments. Make your students' jaws drop with this colour change reaction. Arthur C Clarke's famous third law: 'any sufficiently advanced technology is indistinguishable from magic' can also be applied to chemistry. There's.

"Iodine clock" experiment (the BriggsRauscher reaction) MEL Chemistry
The Briggs-Rauscher reaction is an oscillating clock reaction that cycles through colors. The Briggs-Rauscher reaction is an oscillating clock chemical reaction that cycles from clear, amber, to deep blue several times before finally ending as a blue-black mixture. It is one of the most popular chemistry demonstrations because it's easy and reliable.
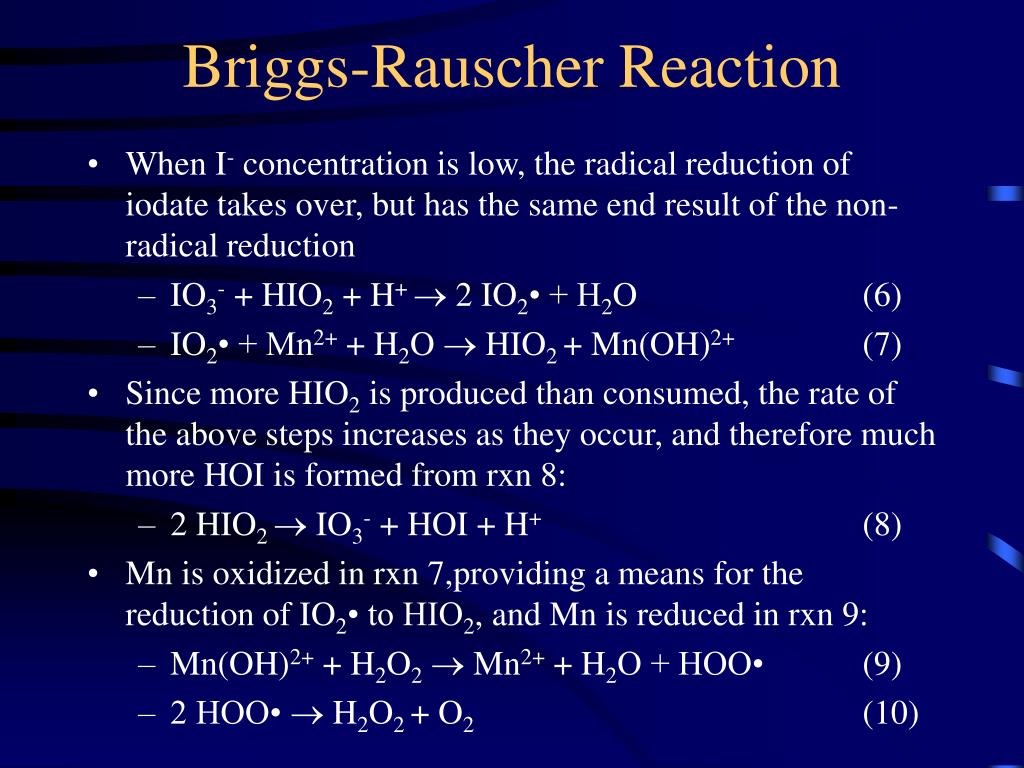
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379
Quenching Analysis of the Permanganate−Hydroxylamine Oscillator. The Journal of Physical Chemistry A 1997, 101 (7) , 1317-1323. DOI: 10.1021/jp962559k. V. Vukojević,, P. Graae Sørensen, and, F. Hynne. Predictive Value of a Model of the Briggs−Rauscher Reaction Fitted to Quenching Experiments. The Journal of Physical Chemistry 1996, 100.

Oscillating reactions Briggs Rauscher BR method YouTube
A Potentiometric Study of the Briggs-Rauscher Oscillatory Reaction in a Solution of Ionic Surfactants and Tetrabutylammonium Hydrogensulphate. ChemistrySelect 2018 , 3 (39) , 10951-10958.

Briggs Rauscher Oscillating Chemical Reaction YouTube
The Briggs-Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking colour changes: the freshly prepared colourless solution slowly turns an amber colour, then suddenly changes to a very dark blue..

BriggsRauscher Oscillating Color Change Villanova College Chemistry Blog
Reaksi Briggs-Rauscher, juga dikenal sebagai 'jam berosilasi', adalah salah satu demonstrasi paling umum dari reaksi osilator kimia. Reaksi dimulai ketika tiga larutan tidak berwarna dicampur bersama-sama. Warna campuran yang dihasilkan akan terombang -ambing antara bening, kuning, dan biru tua selama sekitar 3-5 menit.

BriggsRauscher Reaction MIT Chemistry Behind the Magic YouTube
MIT Chemistry Behind the MagicView the complete course: http://ocw.mit.edu/behindthemagicInstructor: John Dolhuh, Jessica HarropLicense: Creative Commons BY-.

How To Do BriggsRauscher Reaction At Home Diy YouTube
Reaksi Jam Berosilasi atau Briggs-Rauscher berubah warna dari bening menjadi kuning menjadi biru. Siklus reaksi antar warna selama beberapa menit, akhirnya berubah menjadi biru-hitam. Coba Reaksi Perubahan Warna Briggs-Rauscher. Demonstrasi Air Menjadi Darah atau Anggur yang Menyenangkan.
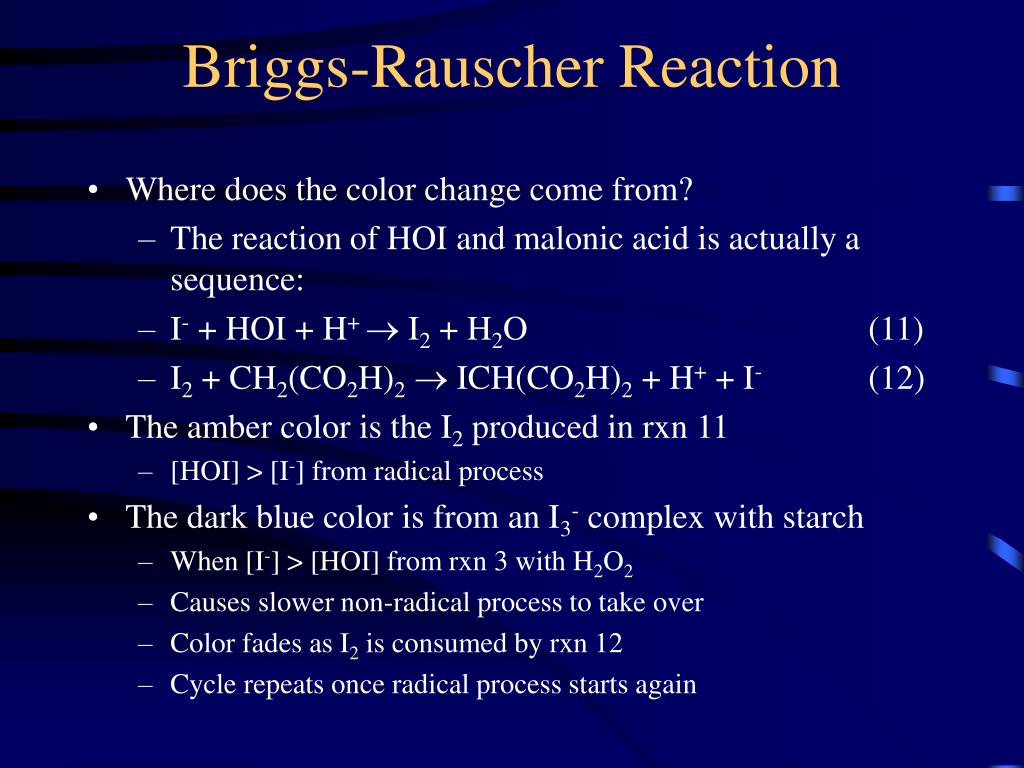
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379
X870: Briggs-Rauscher Reaction- Oscillating Clock . Introduction. Three colorless solutions are combined into a large beaker and the mixture becomes amber, then blue-black, and then colorless again. This sequence of color changes will repeat with a period of approximately 15 seconds at 25 oC. The reaction last about 5 min.

A BriggsRauscher Reaction YouTube
The Briggs-Rauscher reaction, also known as 'the oscillating clock', is one of the most common demonstrations of a chemical oscillator reaction. The reaction begins when three colorless solutions are mixed together. The color of the resulting mixture will oscillate between clear, amber, and deep blue for about 3-5 minutes.

BriggsRauscher Berubah Warna Berubah Reaksi Matematika Ilmu Teknologi 2024
Reaksi Briggs-Rauscher, juga dikenal sebagai 'jam berosilasi', adalah salah satu demonstrasi paling umum dari reaksi osilator kimia. Reaksi dimulai ketika tiga larutan tanpa warna dicampur bersama. Warna campuran yang dihasilkan akan berosilasi antara jernih, kuning, dan biru tua selama sekitar 3-5 menit..

Briggs Rauscher Reaction ChemTalk
Send. The reactions involved in the BriggsndashRauscher BR mechanism are the followingwhere is malonic acidSuppose these reactions take place in an open reactor Then under certain circumstances the color of the solution in the reactor shows regular oscillation between amber and dark blueThis Demonstration simulates the BR mechanism in a flow.